There are more than 200 bones in the human body. Bone is formed during embryonic and postnatal skeletogenesis by two distinct, well-organized processes, intramembranous and endochondral ossification.
Mesenchymal stem cells differentiate into chondrocytes to form a cartilaginous template, which, for long bones, induces bone formation through endochondral ossification. Extracellular signal-regulated kinase 5 (Erk5), which is a member of the mitogen-activated protein kinase (MAPK) family, is phosphorylated by MAPK/Erk kinase-5 (Mek5) to regulate the function of various cell types.
Although the MAPK Erk1/2 pathway is well known for regulating skeletogenesis, the in vivo physiological role of the Mek5/Erk5 pathway in skeletal development has been largely unclear to date, because of the early embryonic lethality of global Erk5 knockout mice.
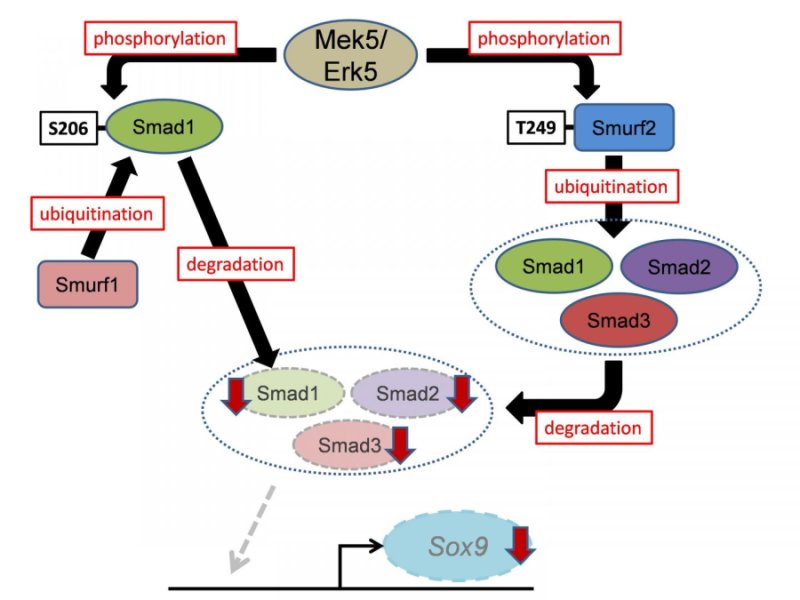 |
Schematic model of this study.
Credit: Kanazawa University
|
By using cell-specific mouse genetics approaches revealed that Erk5 plays a crucial role in skeletogenesis in vivo. Paired-related homeobox 1 (Prx1) is expressed in mesenchymal stem cells in the limb buds. Mesenchymal stem cell-specific Erk5 knockout embryos (Prx1-Cre;Erk5fl/fl embryos) displayed wider long bones and impaired mineralization of the metatarsal. In contrast, chondrocyte-specific Erk5 knockout embryos (Col2a1-Cre;Erk5fl/fl embryos) recapitulated only the wider long bone phenotype of Prx1-Cre;Erk5fl/fl embryos.
Accordingly, the investigators revealed that Erk5 controls.
The study demonstrated that Smads transcriptionally activate the expression in mesenchymal stem cells of sex-determining region within the Y-type high-mobility group box protein 9 (Sox9), which is the principal transcription factor involved in skeletogenesis. Moreover, using mouse genetic rescue experiments, the investigators revealed that Sox9 is a critical mediator of Erk5-dependent skeletogenesis.
In conclusion, the Mek5/Erk5 pathway is critical for skeletogenesis in vivo through its expression in mesenchymal stem cells and modulation of Smad protein stability (Smad1, 2, and 3) via Smurf activity (Smurf1 and 2). These findings improve our understanding of the molecular mechanisms underlying skeletal development and spur the development of drugs targeting human cartilage diseases associated with abnormal chondrocyte differentiation and maturation. Moreover, since the Smurf2/Smads cascade is associated with other diseases, including cancer and ageing, the newly identified Mek5/Erk5/Smurf2/Smads/Sox9 cascade is a candidate target for developing drugs to treat a variety of human diseases.
- Early chondrogenic differentiation of mesenchymal stem cells, including mesenchymal condensation, through its expression in skeletogenic progenitors
- Chondrogenic differentiation after the formation of mesenchymal condensations through its expression in chondrocytes.
The study demonstrated that Smads transcriptionally activate the expression in mesenchymal stem cells of sex-determining region within the Y-type high-mobility group box protein 9 (Sox9), which is the principal transcription factor involved in skeletogenesis. Moreover, using mouse genetic rescue experiments, the investigators revealed that Sox9 is a critical mediator of Erk5-dependent skeletogenesis.
In conclusion, the Mek5/Erk5 pathway is critical for skeletogenesis in vivo through its expression in mesenchymal stem cells and modulation of Smad protein stability (Smad1, 2, and 3) via Smurf activity (Smurf1 and 2). These findings improve our understanding of the molecular mechanisms underlying skeletal development and spur the development of drugs targeting human cartilage diseases associated with abnormal chondrocyte differentiation and maturation. Moreover, since the Smurf2/Smads cascade is associated with other diseases, including cancer and ageing, the newly identified Mek5/Erk5/Smurf2/Smads/Sox9 cascade is a candidate target for developing drugs to treat a variety of human diseases.
We welcome researchers from different part of the to submit abstract on their latest research at our upcoming conference Cell Tissue Science 2019 which is mainly focuses on the complications the consequences of Stem Cell, Regenerative Medicine, Stem Cell Therapy, Cancer Cell Biology , Technical Advancements in cancer treatment and many more.We welcome you to the our upcoming conference “ 12th World Congress on Cell & Tissue Science” . For more info visit :Cell Tissue Science 2019
